
Humans are exposed to alloys daily, from household tools for handiwork to road bridges for essential travel. With their wide range of applications, alloys are essential to both industry and society.
Alloys are mixtures of two or more types of metal that combine the benefits of the parent materials or sometimes generate completely new ones. One type of alloy that possesses unique advantages is the nickel-based alloy, an alloy with nickel as its principal element. Because of their excellent corrosion resistant properties, nickel-based alloys have found applications in a variety of industries including the nuclear industry, petrochemical industry, and aerospace industry, to list just a few.
Although nickel-based alloys exhibit excellent corrosion resistance, corrosion is a naturally occurring process that can only be minimized, not entirely avoided. In this context, understanding the corrosion performance of alloys, determining how alloying elements affect corrosion behaviour, and learning how to properly apply alloys to avoid failure is essential.
After receiving his Bachelor of Science degree with Honours Specialization in Chemistry in 2015, Henderson wanted to turn his research into action.
“The fourth-year research project that students in Chemistry have to go through opened my eyes to research and I ended up doing a research project with another faculty member in the Chemistry Department, and I loved it,” said the Ph.D. candidate.
“I loved research, but I felt for me what was missing was sort of how what I was doing connected to the big picture, or real-world problems. So rather than study fundamental science, I hoped to enter a research program that was more directly related to society.”
Henderson has watched his dreams come to fruition over the past five years, collaborating with world-class scientists, publishing over a dozen articles, and receiving a prestigious NSERC-CGSD scholarship. Now, the chemist is completing a personal and professional milestone: his PhD thesis.
Henderson is writing his final thesis paper on the corrosion of nickel-based superalloys. He’s interested in understanding how individual alloying elements change the behaviour of an alloy in different environments, mainly high chloride environments.
Nickel-based superalloys can contain a large amount of alloying elements, such as chromium, molybdenum, or tungsten. These alloying elements can be added to a body of nickel to increase some of its properties, whether those be corrosion properties, mechanical properties, or both.
“If you know the effect of an alloying element or something that you’re adding to nickel, you can tailor the alloys for specific applications,” Henderson clarified.
Because nickel-based alloys can accommodate a large amount of alloying elements, the alloy can exhibit excellent corrosion resistance. Alloys can form a protective oxide film in a process known as passivation, and this protective film can prevent corrosion to varying degrees. Alloying additions like chromium, for instance, can induce passive behaviour.
This feature is similar to that of a stainless steel, an iron-based alloy. One can add chromium to iron, forming a passive film which is referred to as stainless steel. This passive film reduces the corrosion rate of the metal.
Yet, iron-based alloys can have a two-phase microstructure, or “two different types of atomic arrangements in the same metal.” While this structure gives stainless steel some attractive mechanical properties such as strength, “in the case of corrosion, it’s not always favourable to have a secondary phase,” said Henderson.
This two-phase structure could potentially cause stainless steels to suffer from galvanic corrosion, which is corrosion damage that occurs when two dissimilar materials are coupled. Despite their wide usage, stainless steels may fail in environments that are too aggressive.
Where stainless steels may fail is where Henderson’s nickel-based superalloys come into play.
Nickel alloys are based on the same principle as stainless steels, but one can generally add more alloying elements while maintaining a single-phase structure. As a result, these superalloys can exhibit great corrosion resistance.
“You can generally add more of the sort of elements that you want to add to make it a corrosion-resistant alloy,” the PhD student clarified.
In the nuclear industry, understanding how to properly apply alloys to avoid failure is essential, as failure could potentially cause accidents involving loss of life, environmental damage, or large economic costs.
“In the case of the nuclear industry, in nuclear reactors, they don’t even want the possibility of failure. So, if there’s a risk or there’s a concern that something’s going to fail and stainless steel is not quite enough corrosion resistant, it’ll rely on a superalloy.”
Through his thesis, the PhD candidate wants to learn more about how alloys can resist corrosion under certain environments, so one can better design them for specific uses. To do so, Henderson has had two focuses: investigating how and why corrosion may occur, and investigating how individual alloying elements affect corrosion behaviour in different environments.
It would be much easier for corrosion scientists and engineers to predict the corrosion rate of a material if its entire surface was corroding uniformly; yet, nature often works in mysterious and complex ways. That’s why one of his first focuses was to look at the mechanisms of crevice corrosion.
Crevice corrosion is a form of localized corrosion. It refers to the localized attack on a metal surface at or near the gap or crevice between two joining surfaces.
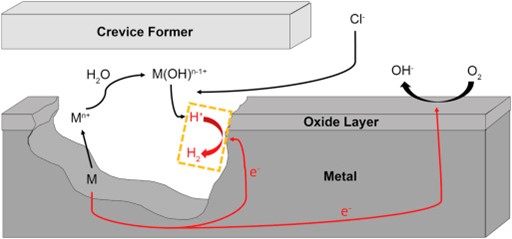
“In the case of nickel-based alloys you have this protective film on the surface. If the protective coating is broken, you would expect the corrosion to occur rapidly where it’s broken,” explained Henderson.
“The nickel-based alloys have very low general corrosion rates, and that’s because they form a film…But because they form a film, you have to be aware of any localized processes that might occur. At that local breakdown, you can penetrate through a material very quickly.”
Crevice corrosion is an important and unintended process that may limit the functionality of a material. After gaining an understanding of why and how crevice corrosion might happen, it was also important for Henderson to look at how the alloying elements change the behaviour of the alloy.
Henderson has developed new techniques with scientists at the University and beyond to reveal the roles of the alloying element additions in nickel-based alloys.
“Part of research is trying stuff, trying new approaches. So, people have studied nickel-based alloys for some time, [but] to continue to get new information, we have to try new things,” said the PhD student.
While Henderson mainly used electrochemical techniques at the beginning of his graduate studies, he turned off the main road and explored the use of new techniques in collaboration with colleagues at Surface Science Western.
Surface Science Western (SSW) is a research laboratory housed at the University, which specializes in analyzing surfaces and materials. With one of Canada’s top-supplied laboratories, the technologies at SSW helped Henderson gain a more holistic understanding of the properties of his nickel-based alloys.
“With the help of Surface Science Western, and with the help of a lot of their surface scientists there, especially…Dr. Mark Biesinger and Brad Kobe, we were able to look at things like X-ray photoelectron spectroscopy, or XPS for short.”
The problems associated with materials can be understood by gaining information about the physical and chemical interactions that occur on a material’s surface. XPS is a well-established technique that provides information from the outermost surface of a sample, giving Henderson insight into the thin oxide film formed on nickel alloys that provides corrosion resistance.
“It’s great for analyzing nickel-based alloys, or any alloy that has a very thin protective film on it,” Henderson claimed.
Yet, Henderson and colleagues have been working to push traditional XPS methods forward.
To learn more about the properties of his nickel alloys, the PhD candidate has worked closely with surface scientists to explore the use of XPS imaging and the use of QUASES (Quantitative Analysis of Surfaces by Electron Spectroscopy), a software program for simulating compositional variations with depth within a sample surface.
Routine XPS imaging has only been around for about two decades, but the technology can help scientists retain spatial information about a surface. This can help them examine the thickness variations of these very thin coatings or even understand the distribution of chemistries across a surface.
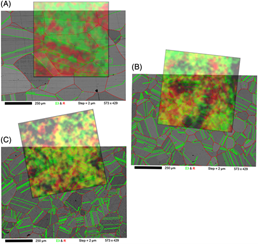
Henderson has also applied QUASES to XPS techniques. QUASES software provides a “tool for quantification” in XPS. It provides information about electron energy loss processes and it has also been used to determine the thickness of a surface film in an extremely small, nanometer range.
While colleagues at the University have helped Henderson explore these relatively new techniques, he has also furthered his exploration beyond Southwestern Ontario.
Two years ago, the PhD student travelled to Paris, France for four months to work with world-class scientists Dr. Kevin Ogle and Dr. Philippe Marcus. With Dr. Marcus, Henderson explored the structure of the oxide film formed on his alloys, and the mechanisms occurring during oxidation, using in situ time-of-flight secondary ion mass spectrometry (in situ ToF-SIMS). Under Dr. Ogle’s supervision, Henderson was also able to investigate the behaviour of the oxide film using a technique called atomic emission spectroelectrochemistry (AESEC).
The Electrochemistry and Corrosion Science group led by Noël and Shoesmith primarily use electrochemistry as a tool to study corrosion.
“It’s kind of tracking electricity, or the flow of electrons, to get an idea of the rate of reaction…That gives us good information on how fast something is going,” Henderson simplified.
But Henderson’s nickel-based alloys are complex materials composed of multiple alloying elements. While electrochemistry is helpful for determining the rate of reaction, it doesn’t necessarily provide information on the behaviour of the individual alloying elements.
That’s where AESEC technology comes in handy.
This technique allows scientists to monitor the dissolution of a large number of elements simultaneously.
“It allowed me to monitor the dissolution of specific alloying elements in real time, or what they call in situ. It allows you to identify exactly what’s dissolving,” the graduate student clarified.
Using these techniques, one of the most significant findings Henderson has made in his PhD surrounded the element molybdenum. He’s been able to investigate the role of this element in stabilizing the protective film on nickel alloys, as well as in preventing localized corrosion.
While Henderson was able to test out some new toys beyond the doors of the University, his findings were brought back to his groupmates in the Chemistry Department at Western.
“The techniques that I’m exploring, for example AESEC or QUASES, could also be applied to other projects in our lab,” Henderson observed.
“Just because I’m developing them for the nickel-alloys doesn’t mean they’re not directly relatable to the other projects…In fact in most cases, they are relatable.”
Currently, Henderson is one of the only members who isn’t working directly on the group’s major project: nuclear waste management.
Researchers at ECS Western are designing systems for the storage of nuclear waste in collaboration with the Nuclear Waste Management Organization (NWMO). Developing methods to safely and permanently dispose of nuclear waste is something of high stakes, as container corrosion could lead to the release of radioactive materials.
While the group is currently investigating copper as the outer shell for a nuclear fuel container, Henderson’s nickel alloys have previously been studied for their potential application in nuclear waste repositories, namely Yucca Mountain, USA. The Yucca Mountain Nuclear Waste Repository project was challenged by the public and many political figures, and funding for the site ended under the Obama Administration in 2011.
Although nickel-based alloys are not being used in the group’s largest project, these alloys have previously been considered a viable candidate material for the barriers in geological repositories. So, Henderson’s work on how alloying elements affect corrosion behaviour is something of importance to the entire research group.
“So, I’m no longer directly studying nuclear waste containers, but of course, all of the information that we get is important regardless,” Henderson remarked.
“The sharing of knowledge, the sharing of techniques, the sharing of skills between projects is always beneficial.”
People may overlook the role of corrosion in society. But many scientists, engineers, and researchers dedicate their profession to properly designing materials to keep society safe.
“If we improve the understanding of the behaviour of alloys, we can better design alloys for specific uses and avoid failure…That’s exactly what everyone’s after, right? We’re just trying to better understand how things are corroding so we can avoid failure,” Henderson remarked.
After working with scientists from around the world, developing new techniques in his field, and winning numerous awards, the PhD student is nearing the end of his academic career with Noël and Shoesmith’s group.
“Right now, I have one semester left…I’m approaching the light,” the grad student chuckled.
Henderson’s goal before entering the group was to see how his research could lead to action. With a PhD thesis on the way, it seems like his academic career has now come full circle.
“You know, talking to people at conferences who are in the industry, and when you share your results with them and they’re kind of like ‘Oh, I didn’t know that, that’s really good to know’…To me, that’s the cherry on the cake.”
_____________________________________
Graphic Credits:
- Henderson, J.D.; Ebrahimi, N.; Dehnavi, V.; Guo, M; Shoesmith, D.W.; Noël, J.J. The role of internal cathodic support during the crevice corrosion of Ni-Cr-Mo alloys. Electrochimica Acta [Online] September 1, 2018, 1600-1608. ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0013468618315408.
- Kobe, B.; Badley, M.; Henderson, J.D.; Anderson, S.; Biesinger, M.C.; Shoesmith, D. Application of quantitative X-ray photoelectron spectroscopy (XPS) imaging: investigation of Ni-Cr-Mo alloys exposed to crevice corrosion solution. Surface and Interface Analysis [Online] October 16, 2017, 1345-1350. Wiley Online Library. https://onlinelibrary.wiley.com/doi/abs/10.1002/sia.6325