We rely on our bodies to perform the necessary functions of life, from walking and breathing to processing food or fighting infection. The human body can do some remarkable things, but like any machine, it may require maintenance if it begins to break down – what happens when our hips start to hurt after a morning jog, or our tooth falls out biting into an apple?
Titanium, a metal known for its strength, lightness, and resistance to corrosion, is the most popular material for medical and dental implants in Canada – it is not harmful or toxic to living tissue, and the metal can also withstand corrosion from harsh bodily substances. One can find this versatile metal in a variety of applications, from elbow, shoulder, knee or hip replacements to pacemakers and medical equipment.
Surgery isn’t a fun experience for anyone, but after it’s over, we would all like to recover safely. With modern surgical techniques and materials, knee or hip joint replacements should last at least 10 to 20 years, or even our entire lives – but to avoid coming in for another operation, these new metal parts must survive the test of time.
Enter Master’s student Hunter Feltham, who studies with the Electrochemistry and Corrosion Science Group (ECS) and the Nanophysics Laboratory at Western University. He investigates the thin film oxide growth mechanism of titanium – this protective oxide layer protects the metal from any substances which may accelerate corrosion. “What makes titanium so great for things like biomedical implants is its outer protective layer, which grows naturally on the metal in the presence of oxygen and provides a lot of structural integrity,” explained Feltham. “If we understand how this protective film grows, we can create new oxide layers that are better tailored to specific applications.”
Most metals form oxide layers when they are exposed to the atmosphere, but the nature of this protective film depends on the metal and the conditions under which it has been oxidized. For instance, when iron is exposed to moisture or oxygen, it is converted to iron oxide, which has a red, flaky appearance – if left unaddressed, this rust substance can spread, flake off, and compromise the physical integrity of the material. However, when a similar process occurs on titanium, the oxide layer grows uniformly across the entire metal surface and has a consistent thickness – this film also adheres well to the metal, providing a hard, protective layer that separates any corrosive substances from the underlying titanium.
Consider that our blood is similar to saltwater, a substance which is known to accelerate corrosion. If a biomedical implant were to be made out of iron or steel, the main concern would be how fast these metals would deteriorate under these conditions – fortunately, the protective oxide layer on titanium helps it withstand the harsh bodily environment.
“The protective film on titanium can weather almost all conditions, because it is incredibly insoluble and thermodynamically stable,” Feltham elaborated. “Even in a lot of traditionally corrosive environments, it grows thicker and provides more and more protection.”
However, like anything else, titanium isn’t perfect – although the natural oxide layer acts as a protective shield, it continues to grow very slowly in our bodies. As it expands, understanding what this layer is going to incorporate from our bodies, as well as what it will put into our bodies, is crucial to determine before a titanium implant is used in a practical application.
“Theoretically, if chlorine or any other substances were incorporated into the titanium oxide as the oxide film grows in our bodies, it could form titanium chloride, which would have different effects as a protection layer as opposed to simply titanium oxide,” Feltham explained. “Even if there is slow oxide growth, knowing exactly what it is absorbing or putting into the body will help us determine how we can manufacture titanium to last longer and require less replacements.”
For his research, Feltham wishes to determine which underlying growth mechanism is generating the new oxide film, as well as the location in which the film is being formed. Both of these factors will have different implications if scientists or engineers want to manufacture a better oxide coating or if they want to know the long-term effects of having titanium oxide in the body.
There are two leading theories which have been used to explain the growth mechanism of an oxide film, which are the high field model and the point defect model. In both cases, titanium atoms are on one side and oxygen atoms are on the either side of a titanium oxide layer, which is in between.
In the high field model, titanium atoms will push their way through the titanium oxide layer to reach an oxygen atom on the other side, while oxygen atoms will move through the oxide layer to reach the titanium atoms. When these atoms join together on either side, they generate a new oxide layer there – however, researchers need to determine how many atoms have joined together on each side to generate this oxide film to distinguish where it is growing. Since the titanium and oxygen atoms tend not to meet in the middle of the existing oxide layer, the new oxide film could continue to grow on both sides.
In the point defect model, the titanium and oxygen atoms do not push through the oxide film to the other side. Rather, a titanium atom that joins in the middle of the oxide film will take an oxygen atom that is already there – the titanium atom that was left alone will proceed to take another oxygen atom present within the oxide film. This process continues until the last titanium atom left alone in the oxide film takes an oxygen atom that is on the other side of the film, meaning the oxide layer grows thicker – however, these atoms are not coupled up. In fact, if another titanium atom joined the oxide film, the titanium atom that is left alone would have to get a new oxygen atom from the other side of the layer again. This eventually creates ‘point defects’, meaning the structure is missing atoms in specific areas where they should be present, and adjacent atoms will then fill in these missing spaces. Even though no atoms crossed between the oxide film, the titanium or oxygen atoms who are left without a ‘partner’ were initially located on one side of the film, but ended up on the other side during their quest to find a partner – now, the challenge is determining where the new oxide film is growing, because you will not find these atoms coupled together. As a result, the oxide film will continue growing on both sides of the oxide film: between the oxide and the outer environment or the oxide and the bare metal interface.
Determining which growth mechanism is occurring can be quite challenging, because all of the titanium atoms look the same, as do the oxygen atoms. Additionally, these new oxide films are virtually indistinguishable from the protective layer that forms naturally on the metal surface. The natural oxide layer found on titanium is also extremely thin, ranging from five nanometers to seven nanometers – consider that one nanometer is one millionth of a millimeter.
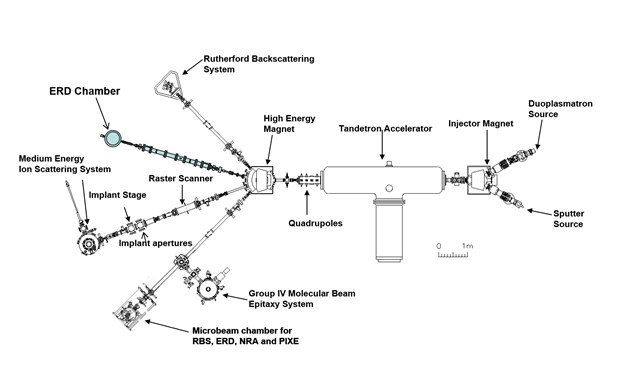
Fortunately, a Tandetron facility housed at the University can be used to investigate samples of titanium on a tiny, nanometer scale. The tandetron is a device that uses high voltages to create intense electric fields that attract and repel electrically charged ions (such as gold), to accelerate them to very high velocities and crash them into a target (such as a titanium layer), where they embed themselves inside of this target. Feltham uses this technology to determine the depth profile of titanium as well as the very slight changes in oxide growth.
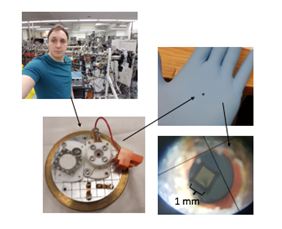
Using this device, Feltham also embeds gold atoms inside of his titanium samples which act as a ‘marker layer’, giving him a reference point to discern where various atoms are moving within the titanium metal. As Feltham increases the thickness of the oxide layer, the titanium metal will begin to ‘shrink’ – since he cannot see all the way through his samples, the gold layer acts as a marking point, allowing him to discern the distance in which the oxide layer has grown relative to a certain area. This is similar to marking the height of a pond; although you cannot see through to the bottom of the water, one can use a marker to determine how deep the pond is and how much the water levels have changed.
Using a variety of electrochemical techniques, the Master’s student also accelerates the corrosion on the surface of titanium to determine how the passive film would perform in a real-life scenario, without having to wait months for limited results. To accelerate corrosion, Feltham fills an electrochemical cell with saltwater and uses a potentiostat, a controllable electrical power supply, to apply voltage to his titanium samples, which he runs through the saltwater solution. The potentiostat can also be used with other measurement systems, such as a frequency response analyzer, to determine the different properties of the oxide films, including their capacitance, impedance, or resistance, all of which are useful for determining which oxide growth mechanism is occurring.
Currently, Feltham is adding a new component to the Tandetron facility to allow him to better analyze the properties of his titanium oxide samples. Ultimately, scientists and engineers could use his discoveries to create new protective coatings in the future – these enhanced layers will help to prevent repair costs, or even threats to safety, where titanium implants are used. For now, Feltham is sharing his discoveries with his lab mates at Western University as they design other materials, such as nuclear fuel containers, that must withstand material decay for generations to come.