Permanently and safely disposing of high-level nuclear waste isn’t a temporary problem – it involves a million-year solution.
Researchers at the Electrochemistry and Corrosion Science Centre (ECS) are working closely with the Nuclear Waste Management Organization (NWMO) to develop a system for the permanent disposal of nuclear waste.
The high-level nuclear waste consists mainly of spent fuel discharged from nuclear reactors. While the fuel’s radioactivity decays away with time, it remains radioactive for up to one million years. Also, the fuel’s chemical toxicity will change little over the lifetime of the Earth; toxic heavy metals such as lead and mercury are poisonous because of their chemical properties, which do not decay away over time.
This makes it crucial to develop a long-term strategy to contain nuclear waste permanently and safely.
In dialogue with over 18,000 Canadians, the NWMO determined that used nuclear fuel should be buried at a depth of 500 m in a deep geological repository (DGR). The proposed multi-barrier disposal system comprises used fuel bundles that will be sealed inside carbon-steel containers covered with a 3 mm thick copper coating. The containers will then be encased in a compacted bentonite buffer box and they will all be buried in suitable host rock in a deep geological repository.

the Department of Chemistry at
Western University
While this multiple-barrier system ensures the nuclear waste will be safely contained, researchers at ECS Western received funding from the NWMO to study the chemical and electrochemical degradation of the copper-coated carbon-steel container when exposed to DGR conditions.
This is a long-term process which could lead to the release of radionuclides into the environment only if container failure occurs.
“The internationally adopted disposal method for the safe containment of Swedish, Finnish, or Canadian high-level nuclear waste is essentially trying to encapsulate them in the steel containers with an outer copper shell or coating as the corrosion protection barrier,” explained Mengnan Guo, a Ph.D. candidate with ECS Western.
“To maintain the longevity and durability of those copper waste containers for many thousands, or even a million years, the corrosion behaviour of copper is a very important phenomenon to study.”
During her Ph.D., Guo analyzed what may be responsible for the long-term and ongoing corrosion of the copper coating for a million years or more: the corrosion of copper induced by sulphide.
Guo joined the Electrochemistry and Corrosion Science group in September of 2016, but she began her graduate journey as a Master’s student in the Physics Department at Dalhousie University in Halifax.
After developing an interest in corrosion science, she participated in an exchange program which allowed her to travel from Halifax, Nova Scotia to London, Ontario to work with the ECS lab at Western University.
“I participated in an exchange program that was offered by Dalhousie University when I was doing my Master’s. It helped me further develop my interest in corrosion science,” Guo recounted.
“While I was [completing] my Master’s study, I decided to try applying to a Ph.D. position in the current lab that I’m working with, and I ended up being very lucky and I got selected.”
The Ph.D. candidate’s work is funded by both the Nuclear Waste Management Organization (NWMO) and the Swedish Nuclear Fuel and Waste Management Company (SKB).
Her work funded by the NWMO has been conducted off-campus with Natural Resources Canada – CanmetMATERIALS located in Hamilton. There, she has been conducting experiments that reflect the sulphide concentrations that would be expected in a Canadian repository and investigates the longer-term behaviour of copper.
SKB is tasked with managing Swedish nuclear and radioactive waste, and they are currently in the process of applying for a license to build and operate a repository for the Swedish nation. Some of Guo’s work provides answers to questions raised during public hearings on licensing and various other considerations of the Swedish nuclear regulatory body and the Government of Sweden. She has mostly completed experiments in the lab at Western associated with higher sulphide concentrations and the relatively short-term behaviour of copper.
For the waste management project the ECS lab is currently working on, Guo and her labmates are investigating different projects which could potentially cause the degradation of the copper coated used nuclear fuel container. They have been investigating the corrosion behaviour of copper under various scenarios, and whether they can use this information to predict the corrosion behaviour of the container over the lifetime of disposal, which ideally could be over a million years.
During the initial stages of container emplacement, oxygen would still be present in the DGR. Oxygen is typically considered to be the dominant oxidizing agent that could cause the corrosion of different metals, including copper.
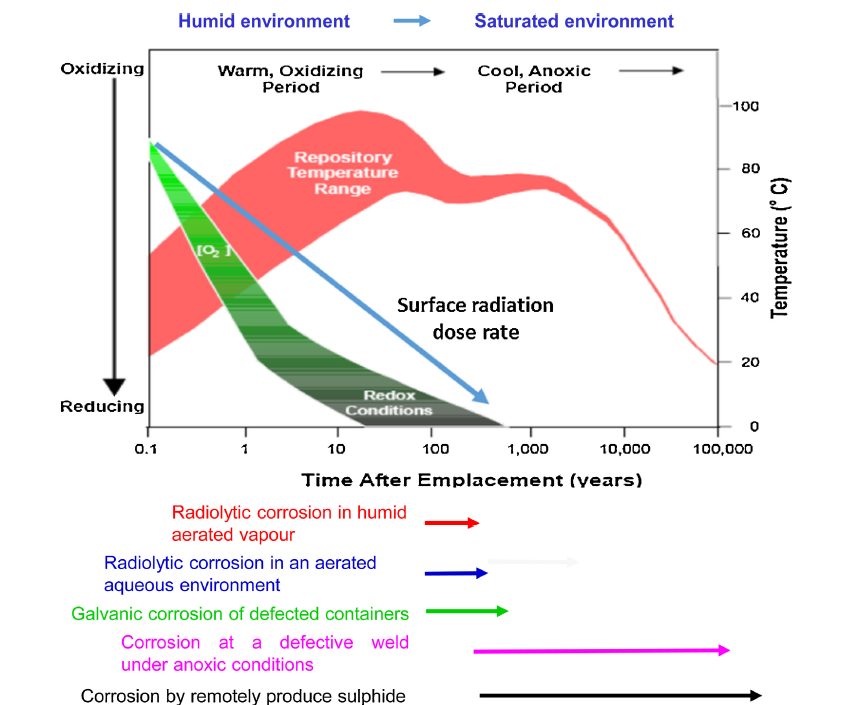
Scientists at NWMO have calculated the inventory of trapped oxygen for the Canadian DGR design geometry, so now researchers can feed this information into their experiments.
Oxygen will only survive in the DGR for a maximum of a few hundred years, a relatively short period of time in comparison to the lifetime of disposal in this waste management program. During the early transient period when trapped oxygen presents in the DGR, copper is not anticipated to be susceptible to severe corrosion.
But after the oxygen has been depleted, what other processes could cause the copper to corrode?
This is where Guo’s work comes in.
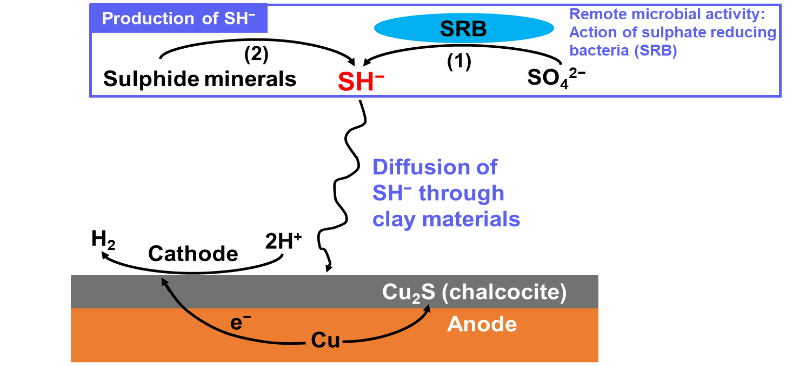
After the entrapped oxygen has been depleted from the DGR, the dominant and long-term threat to the durability of the copper coating would be sulphides. The sulphides are produced by sulphate-reducing bacteria (SRB), which, because of the heat, radiation fields, and highly compacted bentonite clay in the vicinity of the containers, would only be able to produce sulphides at locations remote from the containers. Thus, the sulphide would have to travel a long and difficult path through the compacted bentonite clay before it could reach the container to cause corrosion. This would evidently make this form of corrosion exceptionally slow.
“After a few decades of research, scientists realized that during a specific period of the DGR where oxygen is no longer present, sulphides or sulphide sources may present themselves in the DGR,” the Ph.D. candidate simplified.
“They might have a slight chance to transport themselves from some remote locations in the DGR to the copper waste container surfaces. If that happens, essentially this will be one of the long-term threats to the longevity of copper coated used fuel containers, therefore, this scenario should be investigated.”
While this electrochemical process may cause the copper coating to corrode, this would not inevitably lead to the failure of the container or to the release of radionuclides into the environment.
In Guo’s humble understanding, her work is more of a risk-management project.
“In the actual project of the nuclear waste management, we have more than one barrier to safely contain nuclear wastes,” the graduate student clarified.
“The waste container is just one of the key barriers. In the vicinity of the waste container, essentially, it will be encased in a highly compacted bentonite buffer box.”
Bentonite is a naturally occurring clay-rich sediment containing a mixture of aluminum oxide and silicon oxide, a smectitic clay mineral that has a high cation exchange capacity. The clay material can swell in contact with water, making it an excellent sealing material and a great barrier to slow down groundwater flow, according to the NWMO.
“The presence of the highly compacted bentonite will be the second engineered barrier that could slow down the diffusion of those sulphide species or potentially trap them by forming iron sulphide minerals,” Guo justified.
After the containers are placed in the bentonite clay and buried in impermeable rock underground, it’s very unlikely that radionuclides will escape and be released into the environment. The properties of the bentonite clay would work to isolate most of the radionuclides even if they were to escape from a failed container.
Although Guo is analyzing what would happen in a worst-case scenario, studying the processes that may cause container failure will add certainty to the proposed plan.
To study the corrosion of copper by sulphide, Guo first needs to evaluate the potential corrosion damage of the copper coating. To do so, she uses a sample of copper and a corrosive solution, in this case, an aqueous sulphide solution. Then, she immerses her copper specimens in different concentrations of these solutions.
Guo uses a linear polarization resistance (LPR) test to monitor the corrosion rate of a material. This method can be used to indicate the corrosion resistance of materials in an aqueous environment, or in other words, to determine how fast a metal is oxidized during an experiment. ‘Current’ and ‘resistance’ are the parameters commonly used to reflect the corrosion rate.
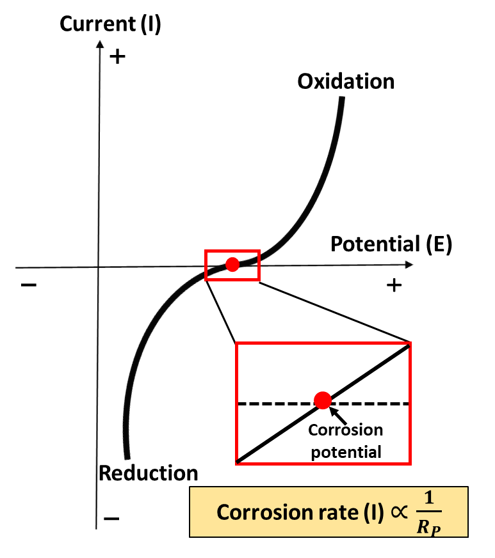
As indicated in the red box shown in the figure above, the potential at the metal surface will be swept in the positive and negative directions in a linear fashion. In this window of conditions, the current-potential relationship is linear and related to the corrosion resistance of the material. Thus, one could use the measured slope of this relationship to represent the corrosion rate of a metal. The corrosion rate is high when the resistance is low (slope is high), while the corrosion rate is low when the measured resistance is high (slope is low).
Sometimes researchers use other features depicted in the data, such as a peak or a plateau, to give them insight into other types of reactions or the properties of a specific corrosion product on the metal.
“We would monitor the corrosion potential of our copper through the immersing experiments as well as the corrosion resistance, or linear polarization resistance,” said Guo.
“We would do so, so that we can evaluate the corrosion rates of the copper based on the measured resistance in the specific corrosive environment.”
After analyzing the corrosion rates and the potential corrosion damage of the copper, Guo would then use various surface analytical techniques to examine the corroded metal. The ECS lab works closely with members of Surface Science Western (SSW), a top-supplied research laboratory housed at the University which specializes in analyzing surfaces and materials.
One of the techniques she uses is scanning electron microscopy (SEM), a well-known surface analytical technique that allows her to analyze the shapes or the morphologies of the corrosion products formed on the metal’s surface. Confocal laser scanning microscopy (CLSM) is another popular surface analytical technique that uses laser light to examine the surface topography of the corroded metal surface.
She has been using a technique called energy dispersive X-ray spectroscopy (EDX) to identify the specific elemental or chemical compositions of the corrosion products found on the copper surface.
Other surface analytical techniques like X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy are used to provide information about the chemical composition of the corrosion products.
In the lab, it’s challenging to replicate the exact repository conditions that would be expected in the DGR.
While doing her immersion experiments, the Ph.D. student uses a higher concentration sulphide solution than what would be expected in the DGR. Doing these experiments can show researchers that the copper coating may be able to maintain integrity even in the most aggressive situations.
“It’s interesting to create aggressive scenarios for our lab scale study, so that if under those aggressive conditions, our metal doesn’t corrode drastically, then we have less concern about more benign conditions anticipated in the repository,” Guo justified.
While these experiments do add confidence to the proposed plan, Guo soon hopes to complete a round of experiments that reflect a more realistic situation that would be expected in the DGR.
“One of my future plans is trying to find appropriate methodologies or techniques to be able to create solution environments containing low sulphide concentrations so that we can further study the more similar corrosion behaviour that would be expected in a realistic DGR,” the graduate student explained.
“This experimental data could perhaps be more useful for those who are doing modelling work to try to predict the long-term corrosion performance of copper for the long-term waste disposal.”
Guo acknowledges that the team isn’t necessarily trying to discover something that nobody else has considered before. Rather, this project tries to use innovative solutions to address real-life problems.
Disposing of used nuclear fuel is something of high risk, so it’s crucial to analyze what would happen in the unlikely event of container failure, because failure, in this case, may jeopardize the safety of human beings and the environment.
“Trying to implement more innovative solutions to these specific, daily-life problems is going to be very important to basically every human being in this country,” said Guo.
“That’s essentially the core value of why we’re doing this research, because it’s a long-term project that should be taken care of for people from different generations.”
For Guo, finding practical solutions to help solve real-world problems is something she cherishes about her work.
“I thought four years would be quite a long time for [continuing] studies,” the graduate student chuckled.
“But it’s actually pretty short, and pretty amazing to be honest…I guess it’s the nature of the work that our lab has been doing because we get to collaborate with industry partners and target the actual daily-life problem solving circumstances.”
Throughout her time with the ECS lab, Guo has had the opportunity to do work in Chemistry, Physics, Engineering, and a variety of other subject areas. Even though she’s finishing up her Ph.D. in the upcoming fall, the grad student doesn’t feel like her journey is over.
She feels like she’s just scratched the surface.
“There are always more things to learn and to study,” Guo remarked.
“I like this current work, I’m passionate about it, and I realize how big this field of study is and how much fun I have learned. So, I would like to continue pursuing a future career within the broad area.”
Graphic Credits:
- Standish, T.; Chen, J.; Jacklin, R.; Jakupi, P.; Ramamurthy, S.; Zagidulin, D.; Keech, P; Shoesmith, D. Corrosion of copper-coated steel high level nuclear waste containers under permanent disposal conditions. Electrochimica Acta [Online] May 21, 2016, 331-342. ScienceDirect. https://www.surfacesciencewestern.com/wp-content/uploads/ea16_shoesmith.pdf.
- Guo, M.; Chen, J.; Martino, T.; Lilja, C.; Johansson, J.A.; Behazin, M.; Binns, W.J.; Keech, P.G.; Noël, J.J.; Shoesmith, D. The nature of the copper sulfide film grown on copper in aqueous sulfide and chloride solutions. Materials and Corrosion [Online] June 5, 2020, 1-8. Wiley Online Library. https://onlinelibrary.wiley.com/doi/epdf/10.1002/maco.202011710.